The 'EVAluation Biomarkers in VTE study' (EVA)
DVT or a pulmonary embolism
In 2016 and 2017 the cardiovascular study group in primary care of the Julius Center (UMC Utrecht) did research on new D-dimer tests together with general practitioners (GPs) and a large number of laboratories.
Using the point-of-care test, GPs can rule out thrombosis in their own practice, contributing to meaningful, cost-efficient and patient-friendly care.
Introduction uitklapper, klik om te openen
New generation of equipment
The laboratory development concerns small, portable devices to measure D-dimer as well as CRP, HbA1c and troponin. Some hospitals already use these devices in some departments. The devices provide a major opportunity for application in primary care practices.
Initial validation
Before it can be used by the general practitioner, the D-dimer test must first be checked for safety and effectiveness (validation). This cannot be done in the laboratory, as there is no gold standard for comparison. Participation of primary care practices and patients is indispensable.
Join in!
The EVA study requires little time from the general practitioner and is hardly taxing for the patient. It is important to consider EVA where appropriate.
Request a participation kit.
Why this study? uitklapper, klik om te openen
In half of patients with potential DVT or pulmonary embolism, the primary care provider can rule out this diagnosis in the consulting room. This requires decision rules (DVT and pulmonary embolism Standard of the Dutch College of General Practitioners) and a point-of-care D-dimer test.
One commonly used POC D-dimer test has been withdrawn from the market because there were doubts about its reliability. The decision to refer a patient for further examination now requires a laboratory test.
The EVA study was started with a view to reintroducing the D-dimer test to general practices. EVA is the acronym for EVAluation of biomarkers in DVT research.
EVA validates all reasonably available POC D-dimer tests for general practitioners. Special emphasis is on POC laboratory devices that can be used for CRP testing in addition to D-dimer measurement.
The ultimate diagnosis of suspected DVT or pulmonary embolism is necessary for a correct assessment. The study can, therefore, only be conducted by general practitioners, not in the laboratory.
Objectives uitklapper, klik om te openen
The objective of the EVA study is to determine the safety and effectiveness of the available POC D-dimer tests to rule out DVT and pulmonary embolism in general practice.
EVA study flowchart uitklapper, klik om te openen
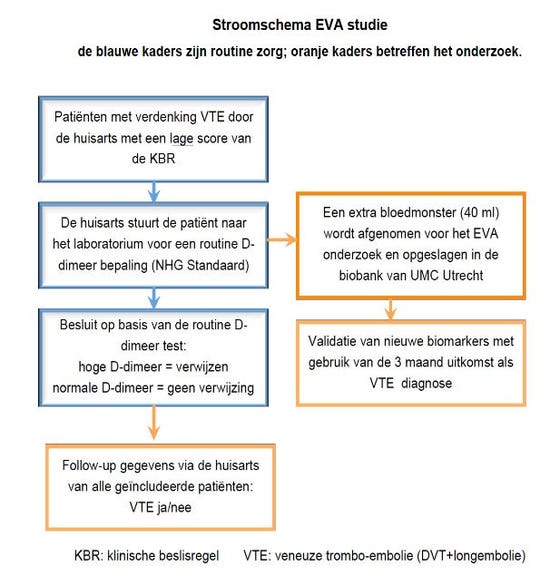
What do the researchers expect general practitioners to do? uitklapper, klik om te openen
- In the event of suspected DVT or pulmonary embolism, the score of the relevant decision rule is decisive (see standard of the Dutch College of General Practitioners or the EVA working form). In the event of a high score, you must immediately refer the patient, without D-dimer measurement. In the event of a low score, you must have a D-dimer measurement carried out by the laboratory.
- You must ask the patient's consent for additional blood sampling (using the same needle) for D-dimer testing for use in general practice.
- You must fill in the practice address, the date of the consultation and the patient's date of birth on the EVA working form.
- You must have the patient sign for approval on the back of the working form.
- You must give the envelope with patient information, sampling tubes and the completed working form to the patient for delivery to the laboratory.
Deelnemende laboratoria uitklapper, klik om te openen
- Certe Groningen
- Certe Leeuwarden
- Antonius ziekenhuis Sneek
- Certe Heerenveen
- Nijsmellinghe Drachten
- Treant Hoogeveen
- Medlon Enschede
- Isala Zwolle
- StJansdal Harderwijk
- Meander MC Amersfoort
- Saltro Utrecht
- Langeland ziekenhuis Zoetermeer
- Star Rotterdam
- SHO Velp
- Jeroen Bosch ziekenhuis
- Zuyderland Sittard
Documents uitklapper, klik om te openen
For more information, send an email to: eva@umcutrecht.nl
